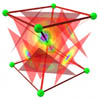
To learn more about the metallic bond of alkali metals, see Chapter 19 in Volume 2 of the book.
Alkali MetalsThe Flash animation may take a moment to download. Note: extended solids are not available in Millsian software.
Alkali Metals (Li, Na, K, Rb, and Cs )
Calculated and experimental values for the lattice parameters and lattice energies for the metallic bond of alkali metals. Calculations are made from exact, closed-form equations containing physical constants only.
1. D. R. Lide, CRC Handbook of Chemistry and Physics, 86th Edition, CRC Press, Taylor and Francis, Boca Raton, (2005-6), pp. 12-15 to 12-18. 2. D. R. Lide, CRC Handbook of Chemistry and Physics, 86th Edition, CRC Press, Taylor and Francis, Boca Raton, (2005-6), pp. 5-4 to 5-18. |
||||||||||||||||||||||||||||||||||||||||||||||||||||