To learn more about the metallic bond of alkali metals, see Chapter 19 in Volume 2 of the book.
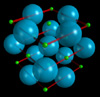
Alkali HydridesThe Flash animation may take a moment to download. Note: extended solids are not available in Millsian software. Potassium Hydride (KH)
Alkali Hydrides (LiH, NaH, KH, RbH, and CsH)
Calculated and experimental values for the ion separation distances and lattice energies for the ionic bond of hydrides of lithium, sodium, potassium, rubidium and cesium. Calculations are made from exact, closed-form equations containing physical constants only.
References: 1. W. M. Muller, J. P. Blackledge, G. G. Libowitz, Metal Hydrides, Academic Press, New York, (1968), pp. 217-229. 2. J. C. Bailar, H. J. Emeleus, R. Nyholm, A. F. Trotman-Dickenson, (Editors), Comprehensive Inorganic Chemistry, Pergamom Press, (1973), p. 401. |
||||||||||||||||||||||||||||||||||||||||||||||||