To learn more about the metallic bond of alkali metals, see Chapter 19 in Volume 2 of the book.
Allotropes of Carbon
Go to: Diamond | C60 Fullerene | Graphite
Diamond
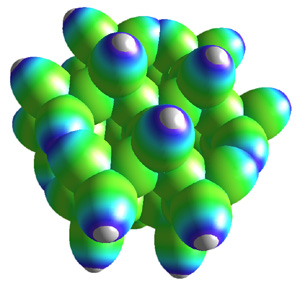 |
The tetrahedral crystal structure of diamond shown with 51 carbon atoms. Click to enlarge. Note: Extended solids are not available in Millsian software. |
Below are calculated and experimental values for select parameters. Calculations are exact, closed-form solutions containing physical constants only.
| Calculated | Experimental | |
| C-C Bond length (a0) | 1.53635 | 1.54428 [1] |
| Total Energy (eV) | 3.74829 | 3.704 [2] |
| CbCaCc Bond Angle (°) | 109.5 | 109.5 [2,3] |
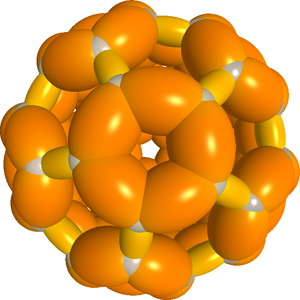
C60 Fullerene
|
The C60 fullerene has been solved in closed-form using Millsian theory. The 3D model is included in Millsian software. Click to enlarge. |
C60 has 60 carbon atoms and 90 bonds, 60 of which are single bonds, and 30 of which are aromatic double bonds. It has 20 hexagonal structures and 12 pentagonal structures. The pentagonal structures contain the single bonds, and and the bridging bonds between pentagonal structures contain the double bonds.
Below are calculated and experimental values for select parameters. Calculations are exact, closed-form solutions containing physical constants only.
| Calculated | Experimental | |
| C-C Bond length (a0) | 1.45345 | 1.455 [4] |
| C=C Bond length (a0) | 1.39140 | 1.391 [4] |
| C60 Total Bond Energy (eV) | 419.75539 | 419.73367 [5] |
| C=C-C Bond Angle (°) | 120.00 | 120.00 [6] |
| C-C-C Bond Angle (°) | 108.00 | 108.00 [6] |
Graphite
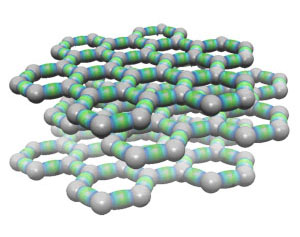 |
The structure of graphite, shown with layers of planes. Click to enlarge. Extended solids are not available in Millsian software. |
Below are calculated and experimental values for select parameters. Calculations are exact, closed-form solutions containing physical constants only.
| Calculated | Experimental | |
| C=C Bond length (a0) | 1.39140 | 1.42 [7] |
| Total Energy (eV) | 4.91359 | 4.89866 [8, 9] |
| CCC Bond Angle (°) | 119.33 | 120 [10] |
References:
1. D. R. Lide, CRC Handbook of Chemistry and Physics, 86th Edition, CRC Press, Taylor & Francis, Boca Raton, (2005-6), p. 4-150.
2. D. R. Lide, CRC Handbook of Chemistry and Physics, 86th Edition, CRC Press, Taylor & Francis, Boca Raton, (2005-6), p. 5-18; 5-45.
3. See http://newton.ex.ac.uk/research/qsystems/people/sque/diamond/
4. W. I. F. David, R. M. Ibberson, J. C. Matthewman, K. Prassides, T. J. S. Dennis, J. P. Hare, H. W. Kroto, R. Taylor, D. R. M. Walton, Crystal structure and bonding of C60, Nature, Vol. 353, (1991), pp. 147-149.
5. D. R. Lide, CRC Handbook of Chemistry and Physics, 86th Edition, CRC Press, Taylor & Francis, Boca Raton, (2005-6), p. 5-42.
6. J. M. Hawkins, Osmylation of C60: proof and characterization of the soccer-ball framework, Acc. Chem. Res., (1992), Vol. 25, pp. 150-156.
7. J. -C. Charlier, J. -P. Michenaud, “Energetics of multilayered carbon tubles,” Phys. Rev. Letts., Vol 70. No. 12, (19930, pp. 1858-1861))
8. M. W. Chase, Jr., C. A. Davies, J. R. Downey, Jr., D. J Frurip, R. A. McDonald, A. N. Syverud, JANAF Thermochemical Tables, Third Edition, Part II, Cr-Zr, J. Phys. Chem. Ref. Data, Vol. 14, Suppl. 1, (1985), p. 536.
9. M. C. Schabel, J. L. Martins, “Energetics of interplanar binding in graphite,” Phys. Rev. B. Vol 46, No. 11, (1992), pp. 7185-7188.
10. D. R. McKenzie, D. Muller, B. A. Pailthorpe, “Compressive-stress-induced formation of thin-film tetrahedral amorphous carbon,” Phys. Rev. Lett., (1991), Vol. 67, No. 6, pp. 773-776.